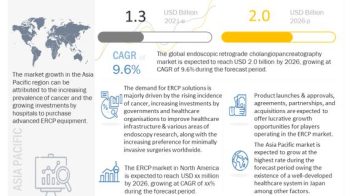
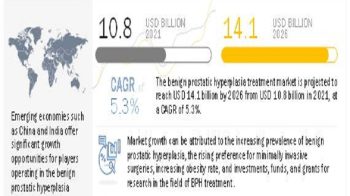
The global sinus dilation devices market is expected to reach USD 2.78 billion by 2023 from USD 1.80 billion in 2018, at a CAGR of 9.1%. The growth of this market is mainly driven by the high prevalence of chronic sinusitis, growing preference for minimally invasive procedures, benefits of balloon sinuplasty/sinus dilation over conventional sinus surgeries, and favorable reimbursement scenario for sinus procedures in developed countries.
Agreements, collaborations, partnerships, and expansions are some of the major growth strategies followed by key players between 2015 and 2018. Key players in the sinus dilation devices market include Entellus Medical Inc. (Subsidiary of Stryker) (US), Acclarent Inc. (Subsidiary of Johnson & Johnson) (US), Medtronic plc (Ireland), Smith & Nephew plc (UK), Intersect ENT Inc. (US), and Olympus Corporation (Japan). Other players in the market include Meril Life Sciences Pvt Ltd. (India), SinuSys Corporation (US), InAccel (India), Jilin Coronado Medical Ltd. (China), and Creganna Medical (Ireland).
In 2017, Acclarent (US) was the leading player in the global sinus dilation devices market. The company’s leading position is attributed to its wide presence in key markets such as the US, Europe, APAC, and the Middle East. The company has a vast customer base spanning more than 160 countries and operates in over 370 locations across the globe. The company has a vast product portfolio and specializes in minimally invasive ENT surgery products. It offers a broad range of devices such as balloon sinuplasty systems, ethmoid punch, and airway dilation systems. To maintain its position in the market, Acclarent spends exhaustively on research and development. In 2016, the company spent 12.7% of its revenue (USD 9.10 billion), which was increased to 13.7% of its revenue (USD 10.5 Billion) in 2017.
In 2017, Entellus (US) held the second position in the sinus dilation devices market. This can be attributed to the strong brand name in high-growth geographies and an increasing presence in emerging markets. The company is present in key regions such as North America and focuses on indirect distribution in 15 countries in Europe. The company has a broad product portfolio in the sinus dilation devices market. It also has an extensive sales force in the US to ensure an increased penetration of its product line in the country. The company also focuses on product launches and product approvals to increase its customer base. For example, in November 2015, the company received FDA Clearance for XprESS Multi-Sinus Dilation System in pediatric patients.
Download PDF Brochure: https://www.marketsandmarkets.com/pdfdownload.asp?id=64349810