Global Active Pharmaceutical Ingredient Market Dynamics
DRIVER: Adoption of organ on chip models in drug development
Despite massive investments in pharma R&D, there has been a direct decrease in the annual number of new FDA-approved drugs—almost directly proportional to the increase in R&D spending. To curb the high cost of drug development, there is a growing need to develop predictive tissue models using human cells to determine drug efficacy and safety in advance of clinical testing.
In this regard, the organ-on-a-chip technology is expected to fill the gaps in drug screening by offering predictive human tissue models. This technology has the potential to revolutionize the pharmaceutical industry by making drug development faster, cheaper, and successful. Thus, the adoption of organ-on-chip models in drug development is considered as a positive indicator of the growth of the APIs market.
COVID-19 impact on the active pharmaceutical ingredient market
With the WHO declaring the COVID-19 outbreak a pandemic, a mix of established pharmaceutical and biopharmaceutical companies, as well as small startups, have stepped forward to develop treatments that target the infection. In just a few weeks, scientists found a list of molecules that target COVID-19. Currently, around 155 molecules are under clinical investigation, and 45 molecules are under preclinical development to be targeted against COVID-19.
In this list, there are four promising drugs (Remdesivir, Chloroquine & Hydroxychloroquine, Lopinavir & Ritonavir, and Lopinavir with Ritonavir plus Interferon beta-1a) that have been repurposed for use against COVID-19. On March 24, 2020, the WHO announced that it had initiated a global mega trial of the four most promising drugs against COVID-19. Countries are in a global race to develop and mass-produce an efficient vaccine to fight COVID-19. The economic and social burden of pandemics has prompted government bodies to increase funding for vaccine development on a global scale.
According to the WHO, as of June 2020, there were over 140 vaccines in various stages of development. Of these, 13 are now in the human clinical trial stage, while others remain at the very early stages of preclinical testing. Increase in funding and research for development of pharmaceutical products will drive growth for the market. However, coronavirus outbreak has disrupted business and economic activities globally.in the first quarter of 2020 It is expected to have a short-term impact on the active pharmaceutical ingredients market to a certain extent.
Download PDF Brochure@ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=263
Expected Revenue Growth:
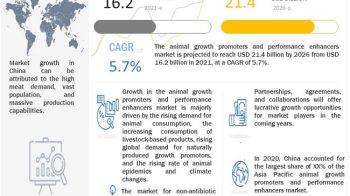
The global active pharmaceutical ingredients market size is projected to reach USD 248.3 billion by 2025 from USD 187.3 billion in 2020, at a CAGR of 5.8% during the forecast period.
RESTRAINT: Unfavourable drug price control policy
Over the last few years, there has been a gradual increase in the regulation of pharmaceutical drug prices globally. The US is a major unregulated market; however, several prominent countries have regulated drug prices. Governments induce price controls to limit spending on pharmaceuticals. However, regulated drug prices mostly result in revenue losses to pharmaceutical companies. These revenue losses lead to a reduction in global R&D spending, which results in fewer new molecular entities (NMEs) being developed per annum.
Such price control policies that reduce pharmaceutical revenues may provide modest relief to individual purchasers; however, they pose risks to long-term innovation in the pharmaceutical industry. For instance, the National Pharmaceutical Pricing Authority (NPPA) of India enforced the Drugs Price Control Order (DPCO) Act in 2013, which empowered it to fix a ceiling on the retail price of drugs. Since then, the list of drugs under price control has steadily expanded from 74 in 1995 to nearly 860 by 2019.
OPPORTUNITY: Highly potent active pharmaceutical ingredients
Highly potent active pharmaceutical ingredients (HPAPIs) represent a significant change in the way pharmaceutical companies are using small molecules to deliver new therapies. The shift toward HPAPIs has led to the development of a pipeline of more effective medicines that require lower doses. The benefits of HPAPIs, such as their high efficacy, lower therapeutic dose requirement (owing to the selective mode of action), and the ability to bind to specific receptors, can be considered as the major factors responsible for their growing demand among manufacturers as well as customers.
The presence of small molecules has traditionally dominated the APIs market. As the generic APIs market continues to get highly competitive, API manufacturers are shifting toward newer avenues such as HPAPIs to differentiate themselves from the competition.
Request Sample Pages@ https://www.marketsandmarkets.com/requestsampleNew.asp?id=263
Recent Developments:
# In 2020, Pfizer (US) signed a multiyear agreement with Gilead Sciences to manufacture and supply Gilead’s antiviral drug (Remdesivir) for the treatment of COVID-19.
# In 2020, Novartis (Switzerland) acquired Aspen’s Japanese operations to strengthen its position in the global generics and off-patent medicines market. Novartis also entered into a manufacturing and supply agreement with Aspen.
# In April 2020, Boehringer Ingelheim (Germany) acquired Northern Biologics, which focuses on therapeutic antibodies targeting the tumor microenvironment. This acquisition broadened Boehringer Ingelheim’s oncology product portfolio.