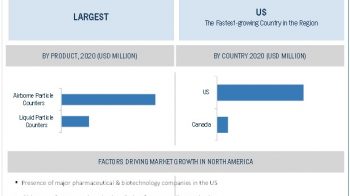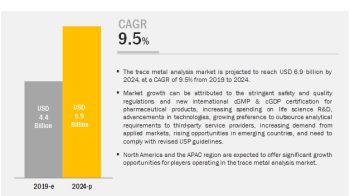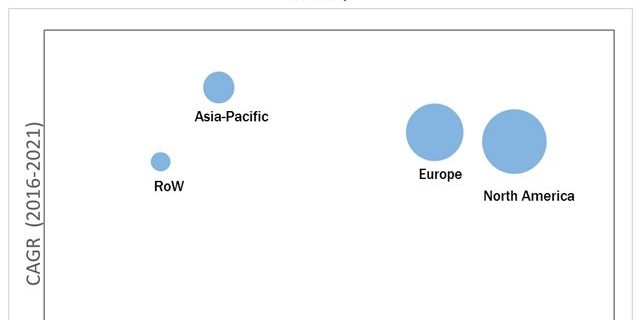
The global analytical laboratory services market, by public health organization is projected to reach USD 333.8 Million by 2021 from USD 202.8 Million in 2016, at a CAGR of around 10.5% during the forecast period. The overall market, by public health organization is positively impacted by factors such as the growing expenditure on drugs and medical devices by public health organizations, government initiatives to strengthen analytical testing capabilities, increasing number of drug approvals & clinical trials, and rising demand for specialized analytical testing services.
Analytical Laboratory Services Market by Public Health Organization, – by types of services (Stability, Raw Material, Physical Characterization, Method Validation, Microbial Testing, Environmental Monitoring, Bioanalytical Testing) – Forecast to 2021
Download PDF Brochure @https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=65590498
On the basis of type of service, the spend assessment is segmented into eight segments, namely, bioanalytical testing, batch release testing, stability testing, raw material testing, physical characterization, method validation, microbial testing, and environmental monitoring.
Target Audience :
⏩ Government Agencies
⏩ Nonprofit Organizations
⏩ Analytical Laboratories
⏩ Private Analytical Testing Providers
Request for Sample Pages @https://www.marketsandmarkets.com/requestsampleNew.asp?id=65590498
Key Market Players :
Food and Drug Administration (U.S.), European Medicines Agency (U.K.), Federal Institute for Drugs and Medical Devices (Germany), Agence française de sécurité sanitaire des produits de santé (France), Agenzia Italiana del Farmaco (Italy), the Spanish Medicines and Health Products Agency (Spain), Central Drugs Standard Control Organization (India), China Food and Drug Administration, and Pharmaceuticals and Medical Devices Agency (Japan).