Biologics are pharmaceutical products that are extracted from biological sources. Biologics safety testing is carried out by manufacturers to detect contaminants like bacterial toxins, mycoplasma, and viruses. It is a mandatory process in these companies for the quality control of raw materials as well as for process control and validation of end products.
What the Market Looks Like?
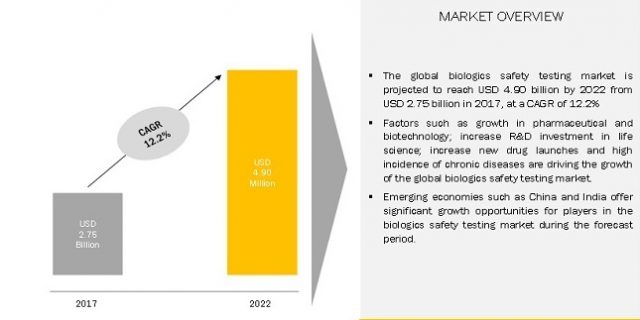
The global biologics safety testing market is expected to reach USD 4.90 Billion by 2022 from USD 2.75 Billion in 2017, at a CAGR of 12.2% during forecast period. North America to account for the largest market size during the forecast period.
The major factors driving the growth of this market are the growth in pharmaceutical & biotechnology industry driven by government support, the positive trend of R&D investment in the life science sector, increasing number of drug launches, and high incidence & large economic burden of chronic diseases. In the coming years, emerging markets and increasing pharmaceutical outsourcing are expected to offer growth opportunities for players in the biologics safety testing market.
Download PDF Brochure @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=34624144
What are Key Trends in the Market?
The growth of the Biologics Safety Testing Market is primarily influenced by the following factors:
- Growth in Pharmaceutical and Biotechnology Industries Driven By Government Support
- Positive Trend of R&D Investments in Life Science
- Increase in Number of Drug Launches
- High Incidence and Large Economic Burden of Chronic Diseases
Vaccine and Therapeutics Development segment is projected to register a higher CAGR during the forecast period. Factors such as the rising prevalence of diseases, increasing initiatives for immunization, and increasing company investments in vaccine development are expected to drive the growth of this market segment.
By test type, endotoxin test segment to record the highest CAGR during the forecast period
Endotoxin test segment is projected to register a higher CAGR during the forecast period. The market is mainly driven by the increase in disease prevalence and growth in the number of drug launches. Moreover, Endotoxin testing is used in cell therapy, monoclonal antibody testing, vaccine testing, gene therapy, and drug development.
Read more about Biologics Safety Testing Industry@ https://www.marketsandmarkets.com/requestsampleNew.asp?id=34624144
North America to account for the largest market size during the forecast period.
North America is expected to account for the largest share of this market. The strong R&D trend in life sciences and growth in pharmaceutical industries will drive the biologics safety testing market in the North American region.
Critical questions the report answers:
- Where will all these developments take the industry in the long term?
- What are the upcoming trends for the biologics safety testing market?
- Which segment provides the most opportunity for growth?
- Who are the leading vendors operating in this market?
- What are the opportunities for new market entrants?