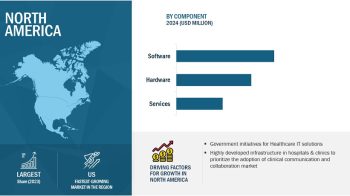
The growth of the global Biosimulation Market can be attributed to factors such as hospital budget cuts, a large inventory of used or old medical devises, rising demand for capital-intensive diagnostic imaging equipment, e-commerce platform enhancing the ease of purchase of refurbished medical equipment, growing preference for eco-friendly products, an increasing number of diagnostic centers & hospitals, and the growing opportunities in emerging economies.
According MarketsandMarkets Research Report – [180 Pages Report] The global biosimulation market is projected to reach USD 2,881 billion by 2022 from USD 1,375 billion in 2017, at a CAGR of 15.9%. Factors such as increase in R&D investments in the pharmaceutical and biotechnology industries, growing adoption of biosimulation software by regulatory bodies, technologically advanced QSP systems, need to curtail drug discovery and development costs, and growth in the biologics and biosimilars markets are driving the growth of the market.
Download PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=838
Market Dynamics
Driver: Growth in the biologics and biosimilar market
Growth in the global biosimilars market is mainly driven by factors such as the growing pressure to curtail healthcare expenditures, growing demand for biosimilars due to their cost-effectiveness, rising incidence of various diseases, increasing number of off-patented drugs, positive outcomes in ongoing clinical trials, and rising demand for biosimilars in different therapeutic applications such as rheumatoid arthritis and blood disorders. As there is very little success in the R&D of new chemical entities, pharmaceutical companies are trying to find new applications for their existing drugs. As toxicity and other vital parameters of drug safety are already tested, biosimulation technologies are used to confirm the hypothesis of using the drugs for a new indication or disease.
Restraint: Lack of standardization
Biosimulation uses a variety of models, tools, and languages for capturing and processing different aspects of biological processes. The current modeling methods do not capture the underlying semantics of biosimulation models to support building, reusing, composing, and merging of varied, complex biosimulation models. The governing bodies are yet to standardize the use of in silico or biosimulation technologies in the drug discovery or development process. Recent technological advancements have increased the computational power of biomedical researchers for building and managing complex biosimulation systems. However, as their models and simulations grow in complexity, researchers find it more difficult to share, manage, and edit their models. The problem of sharing complex biomedical models becomes severe with the lack of scalable standards for model representation and reuse. As a consequence, many model developers are unable to share and build new models upon previously coded models. This shortcoming of biosimulation can only be solved by the provision of a principled, standards-based representation model, which is able to represent biosimulation data unambiguously in both a human and a machine-interpretable way. The biosimulation community, therefore, has a growing need for tools that will help them to efficiently build, manage, and reuse their models.
Opportunity: Emerging applications
The emerging applications of biosimulation in defense, industrial bioprocessing, nutraceuticals, and agri-food production present significant opportunities for the growth of the global biosimulation market. Various biosimulation companies are adopting inorganic and organic growth strategies to expand the applications of their biosimulation software and services. In May 2017, the Institute of Life Science at Swansea University delivered an in silico drug discovery software platform for the UK Ministry of Defense for the development of antimicrobials to meet the needs of defense and security in the country. Likewise, in April 2017, Certara formed a partnership with the Australian Department of Defense. Certara’s d3 medicine company was selected to conduct a national audit to check the research and development capabilities and capacity of Australia’s medical countermeasures (MCM) product.
Challenge: Shortage of biosimulation and modeling experts
Biosimulation uses mathematical equations to represent real-life processes that take place inside of the human body. Because it can replicate human biological elements and their relationships to drugs when they are introduced into the system, researchers are able to simulate a wide variety of scenarios and study the behavior of a human system in different situations.
Request for Sample Pages: https://www.marketsandmarkets.com/requestsampleNew.asp?id=838
Recent Developments
- In April 2017, Simulations Plus signed Distributor Agreement with Quantum Bio Solutions (Q-Bio) (South Korea) To strengthen its relationships with South Korean companies and universities
- In June 2017, Simulations Plus acquired DILIsym Services, Inc. (“DILIsym”) (US). This enabled the company to expand its product portfolio in simulation software and consulting services of drug-induced liver injury.
- In December 2015, Certara acquired XenologiQ (UK) To strengthen its product portfolio in mechanistic pharmacology and modeling and simulation