What This Report
Will Provide?
This study involved four major
activities in estimating the current size of the companion diagnostics market. Exhaustive
secondary research was carried out to collect information on the market, its
peer markets, and its parent market.
The next step was to validate these findings, assumptions, and sizing with
industry experts across the value chain through primary research. Both top-down
and bottom-up approaches were employed to estimate the complete market size.
After that, market breakdown and data triangulation procedures were used to
estimate the size of segments and subsegments.
Expected Revenue Growth:
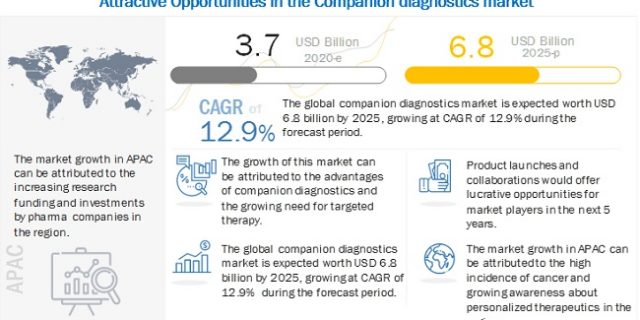
[333 Pages Report] The companion
diagnostics market is expected to reach USD 6.8 billion by 2025 from USD
3.7 billion in 2020, at a CAGR of 12.9%
Major Growth Boosters:
Advantages of companion
diagnostics, the growing need for targeted therapy, the rising importance of
personalized medicine, the increasing global incidence of cancer, and the
ever-increasing application areas of companion diagnostics are driving the
growth of the global companion diagnostics industry. The increasing demand for
next-generation sequencing, the growing significance of companion diagnostics
in drug development, and the rising number of clinical trials are the major
factors driving the growth of this market.
Download PDF Brochure:
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=155571681
Recent Developments:
In June 2020, Thermo Fisher Scientific Inc. and Agios Pharmaceuticals strategic
partnership was made to codevelop a second companion diagnostics platform for
oncology.
In January 2020, QIAGEN N.V. and Amgen collaborated with the aim to develop
tissue-based companion diagnostics for the identification of patients with
cancers that have the KRAS G12C mutation.
In May 2019, QIAGEN N.V. launched the therascreen PIK3CA RGQ PCR Kit in PIQRAY
(alpelisib) therapy in the US to enhance its product portfolio in the companion
diagnostics market.
Regional Growth Analysis:
The companion diagnostics
market in the APAC is estimated to grow at the highest CAGR during the forecast
period. The high incidence of cancer, increasing proteomics & genomics
research, growing research funding, rising investments by pharmaceutical and
biotechnology companies, and growing awareness about personalized therapeutics
in several APAC countries are expected to drive the growth of the APAC market.
Key Questions Addressed in The Report:
1. Who are the top 10 players operating in the global companion diagnostics
market?
2. What are the drivers, restraints, opportunities, and challenges in the companion diagnostics
Industry?
3. What are the opportunities for stakeholders and provide details of the
competitive landscape for key players?
4. What will be growth of companion
diagnostics in North America, Europe, Asia Pacific, Latin
America, and the Middle East and Africa?
Increasing Demand for Next-Generation Sequencing:
NGS-based companion diagnostic
tests aim to unlock molecular information from each patient’s tumor genome to
guide treatment decisions for cancer therapies. Next-generation sequencing
detects multiple biomarkers for multiple drug therapies in a shorter time frame
as compared to other sequencing techniques. The use of NGS panels for biomarker
measurement in one test has the potential to help in the treatment of many
different types of cancers.
Request Sample Report:
https://www.marketsandmarkets.com/requestsampleNew.asp?id=155571681
Key Players:
The companion diagnostics
market is dominated by a few globally established players such as Roche
Diagnostics, Agilent Technologies, Qiagen, Thermo Fisher, and Abbott
Laboratories.