Growth in the global centesis catheters market can be attributed to factors such as the rising target patient population (particularly patients suffering from cancer, TB, and cardiovascular diseases), growing preference for image-guided centesis procedures, and the increasing evidence for the efficacy of centesis procedures for target diseases. However, premium product pricing and complexities associated with centesis procedures are some of the major factors that are expected to restrain the growth of this market in the coming years.
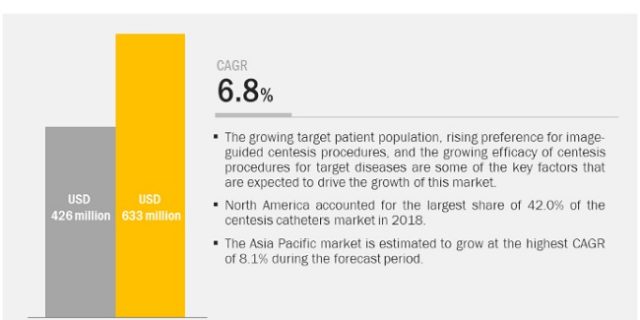
The global centesis catheters market is projected to reach USD 633 million by 2025 from USD 426 million in 2019, at a CAGR of 6.8%. Centesis Catheters Market by Type (Small and Large bore), Procedure (Paracentesis, Thoracentesis, Amniocentesis), Application (Diagnosis, Therapeutics and Palliative care), End user (Hospitals, Ambulatory surgery centers), Region – Global Forecast to 2025.
Based on type, the centesis catheters market is segmented into small-bore and large-bore centesis catheters. The small-bore centesis catheters segment accounted for the largest share of this market in 2018. The large share of this segment can be attributed to the wide availability of small-bore centesis catheters, growing number of target procedures that require small-bore centesis catheters, increasing evidence of the efficacy and safety of small-bore centesis catheters over large-bore centesis catheters, and their higher adoption in diagnostic centesis procedures.
Download PDF Brochure:
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=254850580
Based on procedure, the centesis catheters market is segmented into paracentesis, thoracentesis, arthrocentesis, amniocentesis, and other procedures. The paracentesis segment accounted for the largest share of this market in 2018. The rising incidence of target conditions such as gastrointestinal malignancies, liver cirrhosis, cystic fibrosis, intestinal tuberculosis, chronic liver failure, formation of cysts in the peritoneal cavity, and serious forms of hepatitis is expected to drive the growth of this market segment in the coming years. However, the thoracentesis segment is expected to grow at the highest rate during the forecast period.
Geographically, this market is classified into North America, Europe, the Asia Pacific, Latin America, and the Middle East and Africa. In 2018, North America was the largest regional market for centesis catheters. This can primarily be attributed to the high number of cardiovascular, neurovascular, and other target procedures performed in the region. Factors such as the large patient pool, the presence of a well-established healthcare system, and on-going investments by hospitals to upgrade their operating rooms are driving the growth of this regional market.
The Asia Pacific market is estimated to grow at the highest CAGR during the forecast period. This can be attributed to the presence of a large patient pool, increasing incidence and prevalence of target diseases, improving healthcare infrastructure in Asian countries, and the favorable government initiatives for improving access to healthcare.
Request for Sample Pages:
https://www.marketsandmarkets.com/requestsampleNew.asp?id=254850580
Region Covered in Centesis Catheters Market
The key players operating in the global centesis catheters market are AngioDynamics, Inc. (US), ARGON MEDICAL (US), Avanos Medical Devices (US), Axiom Medical, Inc. (US), B. Braun Melsungen AG (Germany), Becton, Dickinson and Company (US), Blue Neem Medical Devices Pvt. Ltd. (India), Boston Scientific Corporation (US), Canadian Hospital Specialties Ltd. (Canada), Cardinal Health (US), Cook Medical (US), Galt Medical Corp. (US), Guangzhou Leadgem Medical Device Co., Ltd. (China), KM Medical (US), Medical Components, Inc. (US), Medtronic (Ireland), Merit Medical Systems (US), Mermaid Medical A/S (Denmark), MoFlo Medical Technology Co., Ltd. (China), Neuromedex GmbH (Germany), Ningbo Honde Medical Instruments Co., Ltd. (China), PFM Medical, Inc. (US), Polymedicure (India), REDAX (Italy), Rocket Medical plc. (UK), Romsons (India), Smiths Medical (US), Teleflex Incorporated (US), UreSil, LLC (US), and Utah Medical Products, Inc. (US).