Expected Revenue Surge:
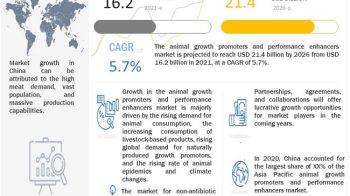
The global Drug discovery services market is projected to reach USD 31.4 billion by 2026 from USD 16.1 billion in 2021, at a CAGR of 14.3% during the forecast period of 2021 to 2026.
Growth Boosters:
Growing R&D expenditure in the pharmaceutical & biopharmaceutical industry, increasing demand for outsourcing analytical testing services, initiatives for research on rare diseases and orphan drugs, and the high cost of in-house drug development are creating new revenue pockets in the drug discovery services market.
On the other hand, stringent regulations governing drug discovery and animal usage are expected to restrain market growth to a certain extent. The shortage of skilled professionals poses a challenge for drug discovery service providers.
Overview of This Study:
This research study involved the extensive use of secondary sources, directories, and databases to identify and collect valuable information for the analysis of the global drug discovery services market. In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, to obtain and verify critical qualitative and quantitative information and assess growth prospects of the market. The global market size estimated through secondary research was then triangulated with inputs from primary research to arrive at the final market size.
Download PDF Brochure@
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=138732129
The chemistry services segment accounted for the largest share of the type segment in the drug discovery services market in 2020.
Based on type, the drug discovery services market is segmented into chemistry and biology services. The chemistry services segment commanded the largest share of this market in 2020. Growth in this market segment is largely due to the widespread application of chemistry in various early drug development phases to deliver robust candidates. The extensive usage of chemistry in academics, biotechnology companies, and large pharmaceutical companies also supports market growth.
The Hit-to-lead identification segment accounted for the largest share of the process segment in the drug discovery services market in 2020.
Based on process, the drug discovery services market is broadly classified into target selection, target validation, hit-to-lead identification, lead optimization, and candidate validation. Hit-to-lead identification segment accounted for the largest share of the drug discovery services market in 2020. Due to its vital role in drug discovery, hit-to-lead identification is the most revenue-generating process, and currently, many CROs are offering these services to pharmaceutical companies.
RESTRAINT: Stringent regulations governing drug discovery and animal usage
Securing safety and efficacy is the prime focus of regulatory authorities during drug approval. Although these approaches help ensure the quality of the products launched in the market, they substantially increase the cost of drug development and the final product. In price-sensitive emerging markets, this factor can significantly impact the uptake of a particular drug. Apart from this, various legislations that ensure the quality of the product (such as GMP) often increase manufacturing costs.
The Asia Pacific region is the fastest-growing region of the drug discovery services market in 2020.
Based on the region, the drug discovery market is segmented into five major regions: North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. The Asia Pacific market is estimated to register the highest growth during the forecast period primarily due to expanding pharmaceutical and biopharmaceutical industry, rising number of CROs, implementation of favorable government policies, increasing number of newly established R&D and manufacturing facilities, and the presence of less-stringent regulations for drug discovery processes (especially in terms of using animals for research) in several APAC countries.
Key Players:
Key players in the drug discovery service Market include Laboratory Corporation of America Holdings (US), Charles River Laboratories International Inc. (US), WuXi AppTec (China), and Thermo Fisher Scientific Inc (US).
OPPORTUNITY: Growth in drugs and biologics market despite Covid-19 Pandemic
Despite the ongoing COVID-19 pandemic, 2020 has been the second-best year for the pharmaceutical industry regarding the number of drugs approved by the US FDA. This year witnessed the authorization of 53 drugs—a number surpassed only in 2018 with 59 pharmaceutical agents. The 53 approvals in 2020 comprised 40 new chemical entities and 13 biologics (of which ten were monoclonal antibodies, two were antibody-drug conjugates, three were peptides, and two, oligonucleotides). The FDA authorized 160 drugs in the last three years (2018–2020), compared to the approval of only 21 drugs in 2010.
Request Sample Pages@
https://www.marketsandmarkets.com/requestsampleNew.asp?id=138732129
This growth in the number of approved products worldwide is attributed to the rising investments by biopharmaceutical companies to develop biologics and biosimilars. More than half of the drug candidates in the discovery stage are biologics, such as proteins, peptides, and monoclonal antibodies. Biologics are expected to contribute around half of the revenue generated by the top 100 pharmaceutical product sales in 2022.