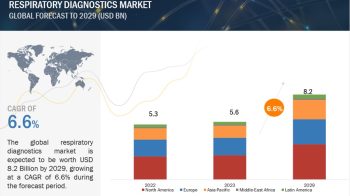
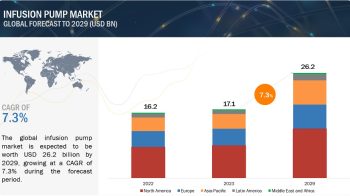
Clinical trials are research studies that involve people. They are used to test new treatments or medicines, or to find out how well existing treatments work for different illnesses and conditions. In a clinical trial, participants receive specific interventions according to the research plan or protocol created by the investigators. These interventions may be medical products
Clinical trials market are research studies that involve people to help test a new medical treatment, device, or drug. These studies are conducted to determine the safety and effectiveness of new treatments, as well as to understand how they compare to existing treatments. Clinical trials are an important part of medical research and a key part of the process of bringing new treatments to market. They are closely monitored and regulated, and involve many different people, including medical professionals, patients, and study sponsors. Clinical trials can also provide important information about a disease or condition, including its causes, symptoms, and natural history.
Overview of Clinical Trials Market
The global clinical trials market is expected to reach a value of USD 67.8 billion by 2026, growing at a CAGR of 8.2% during the forecast period from 2020 to 2026. The increasing prevalence of chronic diseases, technological advancements, and the increasing adoption of clinical trials are the major factors driving the growth of the market. Additionally, the rising demand for personalized medicines, increasing investment in the healthcare sector, and the increasing number of regulatory approvals are also contributing to the growth of the market.
The growth of the market is also driven by the rising number of collaborations between pharma companies, the increasing number of mergers and acquisitions, and the increasing number of government initiatives. However, the high cost of clinical trials, ethical concerns, and the lack of skilled personnel are some of the major factors restraining the growth of the market. Additionally, the emergence of new technologies and the increasing focus on the development of biosimilars are expected to provide major opportunities for the market in the coming years.
A clinical trial is a type of research study in which people volunteer to test treatments, drugs, devices, or other interventions. The goal of clinical trials is to determine the safety and efficacy of these interventions in humans. Clinical trials are used to evaluate new treatments or medications, as well as to discover how diseases and conditions progress and respond to treatment.
Growing Opportunities in Emerging Markets
Emerging markets offer a range of opportunities for businesses looking to expand. These markets tend to be characterized by high growth potential, large populations, and a growing middle class. As a result, they offer the potential for higher returns on investments than more mature markets.
One major opportunity in emerging markets is the potential for growth in consumer spending. As incomes rise and the middle class expands, so does the potential for increased consumer spending. This means that companies selling consumer goods or services in these markets can take advantage of this growth in demand.
Another opportunity in emerging markets is the potential for increased investment in infrastructure. This can include investments in roads, ports, airports, telecommunications, and other projects that can help improve the quality of life in these countries and make them more attractive places to do business.
Benefits of Participating in Clinical Trials
- Access to treatments not yet available to the public. Clinical trials offer patients access to treatments that are not yet available to the public, giving them the potential for improved health outcomes.
- Low-cost or free treatments. Participating in clinical trials provides patients with access to treatments that may otherwise be too expensive for them to afford.
- Improved quality of life. Many clinical trials offer treatments that improve the quality of life for those who are participating.
- Be part of advancing medical research. Participating in clinical trials helps to advance medical research, which can lead to improved treatments in the future.
- Opportunity to access cutting-edge treatments. Clinical trials offer patients the opportunity to try cutting-edge treatments and therapies that may not yet be available to the public.
- Additional medical care. Participating in clinical trials often provides patients with additional medical care, such as regular check-ups, scans, and other tests.
- Improved understanding of the disease. Clinical trials provide patients with an opportunity to learn more about their condition and how it may progress.
- Financial compensation. Some clinical trials offer financial compensation for participating, which can help cover costs associated with the trial or provide additional income for participating families.
Challenges Facing the Clinical Trials Market
- Lack of Patient Engagement: Increasing patient engagement in clinical trials is a challenge that continues to plague the clinical trials market. Patient compliance and recruitment remain the biggest hurdles to successful clinical trials and often result in costly delays.
- Stringent Regulatory Requirements: Clinical trials are heavily regulated, requiring sponsors to adhere to an array of stringent guidelines to ensure patient safety and accurate data collection. These rules and regulations can be complex and expensive to comply with, resulting in delays and additional costs for sponsors.
- High Costs: The cost of clinical trials can be prohibitively high, resulting in fewer studies being conducted and fewer drugs and treatments being approved. The high costs are driven by the need to pay for drug development, research staff, clinical trial sites, and other associated costs.
- Data Quality and Integrity: Ensuring the accuracy and integrity of data collected during clinical trials is a major challenge. Inaccurate data can lead to incorrect conclusions and even dangerous situations for patients taking part in the trial.
- Poor Recruitment Strategies: Poor recruitment strategies can result in inadequate patient enrollment and a lack of diversity in the study population, leading to inaccurate results. Developing effective recruitment strategies that target the right patients is essential to successful clinical trials.
Factors Driving the Clinical Trials Market
- Growing Prevalence of Chronic Diseases: The increasing prevalence of chronic diseases such as cancer, cardiovascular diseases, respiratory diseases, and neurological disorders is one of the major factors driving the growth of the clinical trials market. Clinical trials provide first-hand experience to researchers and medical professionals in understanding the disease and developing new treatments to improve the quality of life of patients.
- Increasing Regulatory Support: Increasing regulatory support from governments and organizations such as the U.S. Food and Drug Administration (FDA) is a key factor driving the growth of the clinical trials market. The FDA has implemented several initiatives to facilitate the development and approval of new drugs and medical devices. These initiatives include the Clinical Trial Transparency Initiative, the Modernization Act, and the Clinical Trials Database.
- Growing Investment in Research and Development: The growth of the clinical trials market is further supported by the increasing investment in research and development (R&D) activities by pharmaceutical and biotechnology companies. Companies are investing heavily in the development of novel treatments and medications to meet the growing demand for new treatments. This is expected to drive the growth of the clinical trials market.
- Technological Advancements: Technological advancements such as wearable devices, data analytics, cloud computing, and artificial intelligence are increasingly being used in clinical trials to improve trial design, reduce costs, and reduce the time required to complete a trial. This is expected to drive the growth of the clinical trials market.
Recent Developments in the Clinical Trials Market
- Rise of Remote Clinical Trials: Remote clinical trials are becoming increasingly popular, as they offer the potential for greater patient participation and reduced costs. These trials rely on technology such as telemedicine, virtual visits, and remote monitoring tools to allow for patient participation from anywhere.
- Growing Use of Artificial Intelligence (AI) and Machine Learning (ML): AI and ML are being used to analyze and process large volumes of data and images, with the aim of improving the accuracy and speed of clinical trial processes.
- Use of Wearable Devices: Wearable devices are being used to monitor patient health during clinical trials, and offer the potential to improve the accuracy of data collected.
- Use of Big Data and Analytics: Big data and analytics are being used to improve the accuracy and efficiency of clinical trial processes, as well as to identify new clinical trial opportunities.
- Adoption of Blockchain Technology: Blockchain technology is being used to improve the security and privacy of clinical trial data, as well as to reduce the cost and complexity of the clinical trial process.
The Future of the Clinical Trials Market
The clinical trials market is expected to continue to grow in the coming years. The increasing demand for new treatments, drugs and therapies due to the global pandemic, as well as the increasing investment in research and development of new treatments, drugs and therapies are driving factors for the clinical trials market. As more companies and research institutes focus on developing effective treatments, drugs and therapies, the demand for clinical trials is only set to increase.
Furthermore, the growing use of technology in clinical trials is expected to revolutionize the clinical trials market. Innovations such as artificial intelligence (AI) and machine learning are expected to improve the accuracy and speed of clinical trials, making them more efficient and cost-effective. Increasing adoption of electronic data capture (EDC) systems, which allow for digital collection and storage of data, is expected to further drive the growth of the market.
Finally, the increasing demand for personalized medicines, which can be tailored to the needs of the individual patient, is expected to continue to drive the growth of the clinical trials market. The increasing use of biomarker-based personalized medicine is expected to significantly reduce the time and cost of clinical trials, thus driving the growth of the market.
Key Takeaways of Clinical Trials:
- Clinical trials are essential for the development of new drugs and treatments.
- Patients should be aware of the potential risks and benefits of participating in a clinical trial.
- Clinical trials must adhere to strict ethical standards to ensure the safety of participants.
- Clinical trials are conducted in phases, with each phase having its own objectives and goals.
- Data from clinical trials is used to determine the safety and effectiveness of new drugs and treatments.
- Clinical trials require extensive planning and coordination to ensure they are conducted properly.
Download PDF Now to Know More about the “Clinical Trials Market Size, Forecast, Growth Opportunities.