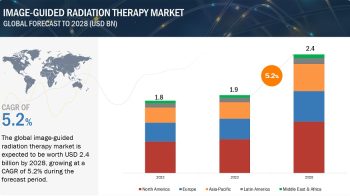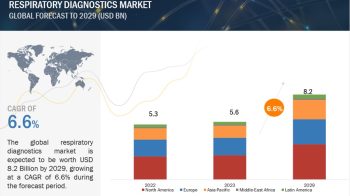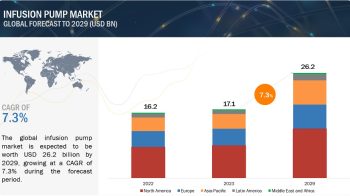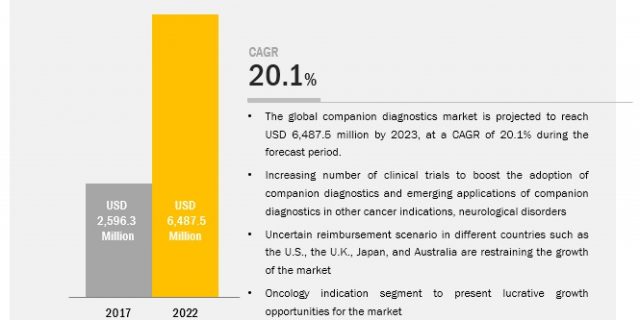
A companion diagnostic assay is an in vitro diagnostic device (IVD) that is used to identify whether a patient with certain diseases could be benefitted by a particular drug through the biomarker assessment.
The key factors driving the growth of this market include the increasing lung cancer cases, growing number of genetic testing, rising need for personalized medicines, and regulatory guidelines that support the companion diagnostics market.
The global companion diagnostics market is projected to reach USD 7.3 billion by 2024 from USD 3.5 billion in 2019, at a CAGR of 15.7%.
Market Dynamics – Drivers
- Advantages of Companion Diagnostics
- Growing Need for Targeted therapy
- Growing Importance of Personalized Medicine
- Increasing Global Incidence of Cancer
- Growing Application Areas of Companion Diagnostics
Polymerase chain reaction (PCR) segment to account for the largest share of the companion diagnostics market, by technology, in 2019
The extractables/leachables testing services segment is expected to dominate the companion diagnostics market in 2019. The large share of this segment can be attributed to the ease of use and widespread availability of PCR kits & reagents in companion diagnostic testing, growing applications of PCR in the high-throughput detection of mutants with a limited or low allele frequency of genes, and high turnaround time of PCR as compared to other technologies.
Download PDF – Companion Diagnostics Market
Pharmaceutical & biopharmaceutical companies are estimated to be the largest end-users of companion diagnostics in 2019
Pharmaceutical & biopharmaceutical companies are expected to account for the largest share of the companion diagnostics market in 2019. The large share of this segment can majorly be attributed to the extensive usage of companion diagnostics in these industries owing to their growing prominence in drug development and the increasing importance of companion diagnostic biomarkers. The increasing demand for personalized medicine as well as the high demand for targeted therapies for various diseases and disorders are also expected to drive the demand and uptake of companion diagnostics among pharmaceutical & biopharmaceutical companies.
Request for Sample Pages – Companion Diagnostic Market
APAC market is estimated to grow at the highest CAGR during the forecast period
The companion diagnostics market in the APAC is estimated to grow at the highest CAGR during the forecast period. The high incidence of cancer, increasing proteomics & genomics research, growing research funding, rising investments by pharmaceutical and biotechnology companies, and growing awareness about personalized therapeutics in several APAC countries are expected to drive the growth of the APAC market.
Leading Companies
The prominent players in the global companion diagnostics market are F. Hoffmann-La Roche AG (Switzerland), Agilent Technologies, Inc. (US), QIAGEN N.V. (Germany), Abbott Laboratories, Inc. (US), Almac Group (UK), Danaher Corporation (US), Illumina, Inc. (US), bioMérieux SA (France), Myriad Genetics, Inc., (US), Sysmex Corporation (Japan), Thermo Fisher Scientific Inc. (US), Abnova Corporation (Taiwan), and Guardant Health, Inc. (US).
View Complete Press Release – Companion Diagnostics Market