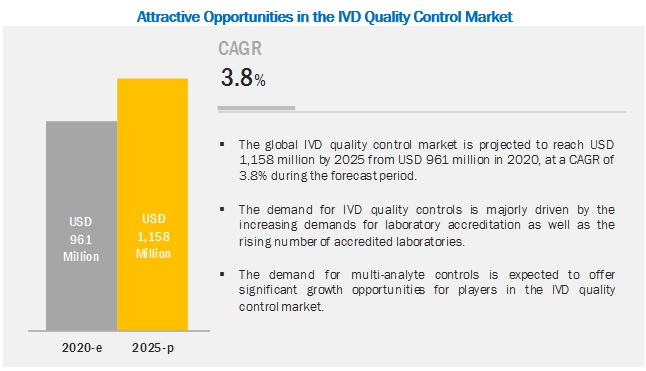
According to research report the global In Vitro Diagnostics Quality Control Market is projected to reach USD 1,158 million by 2025 from USD 961 million in 2020, at a CAGR of 3.8% during the forecast period. The market for IVD quality control is primarily driven by increasing demands for laboratory accreditation, as well as the rising number of accredited laboratories and growing adoption of third-party quality controls. However, the lack of regulations for clinical laboratory accreditation in several emerging countries will challenge market growth.
Download Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=198032582
Based on product and service, the IVD quality control market is segmented into quality control products, data management solutions, and quality assurance services. In 2019, quality control products accounted for the largest share of the global IVD quality control market. Growth in the quality control products market can be attributed to the growth in the number of IVD tests being performed every year along with the increasing number of accredited laboratories.
Based on manufacturer, the IVD quality control market is segmented into third-party controls and original equipment manufacturers (OEMs). In 2019, the third-party controls segment accounted for the largest share of the global IVD quality control market. The large share of this segment can be attributed to the increasing use of third-party quality controls due to their benefits, such as longer shelf-life and flexibility across different reagent lots, which helps reduce costs.
Get Report Sample: https://www.marketsandmarkets.com/requestsampleNew.asp?id=198032582
The market is segmented into five major regions, namely, North America, Europe, the Asia Pacific (APAC), Latin America, and the Middle East & Africa. In 2019, the North America accounted for the largest share of the IVD quality control market. The factors such as recommendations and approvals for quality control products from the FDA and College of American Pathologists (CAP) and the presence of well-established distribution channels as well as leading companies in the US are driving the IVD quality control market in North America.