According to the new market research report “Lateral Flow Assays Market by Application (Clinical Testing (Pregnancy, Infectious Diseases (Mosquito, Influenza, STI, Hepatitis, TB) Cardiac Marker Lipid Test) Veterinary, Food Safety), Product, Technique, End User – Global Forecast to 2025″, published by MarketsandMarkets™.
DRIVERS: High prevalence of infectious disease across the globe;
Despite significant improvements in sanitation and medicine, the global prevalence of infectious diseases is still high. Although non-communicable diseases are the leading cause of morbidity and mortality, infectious diseases remain a major public health concern across the globe. The high prevalence of infectious diseases, such as HIV and malaria, coupled with the underdeveloped healthcare infrastructural facilities and increasing public awareness in developing countries, is expected to drive the adoption of lateral flow assay tests in these countries.
The high prevalence of infectious diseases, such as HIV and malaria, coupled with the underdeveloped healthcare infrastructural facilities and increasing public awareness in developing countries, is expected to drive the adoption of lateral flow assay tests in these countries. In addition, recent outbreaks of infectious diseases from the spread of viruses (such as Ebola, H1N1, and Zika) have highlighted the need for early disease detection capabilities, which is also expected to drive market growth. The current COVID-19 pandemic has resulted in high demand for rapid diagnostic testing through lateral flow technology, supporting the growth of this market in the coming year.
Rapidly increasing geriatric population;
Age-related physiological changes and metabolic inefficiencies often result in chronic diseases such as cystic fibrosis, hepatitis, cardiovascular disorders, and cancer. Geriatric individuals (65 years and above) are more susceptible to these diseases and infectious diseases due to weakened immune systems. With the growing prevalence of chronic diseases, the emphasis on the effective and early diagnosis of diseases is rising. Lateral flow assay techniques are increasingly preferred for disease diagnosis due to their ability to detect symptoms in the early stages. The global rise in the geriatric population, coupled with the increasing patient pool for chronic diseases, translates into higher demand for lateral flow assay tests.
Furthermore, age-caused frailty has been an important concern during COVID-19 management. The risk of death from COVID-19 increases with both age and the presence of co-morbidities such as cardiovascular, pulmonary, or kidney disease, cancer, and obesity.
With the growing prevalence of chronic diseases, the emphasis on the effective and early diagnosis of diseases is rising. Lateral flow assay techniques are increasingly preferred for disease diagnosis due to their ability to detect symptoms in the early stages. The global rise in the geriatric population, coupled with the increasing patient pool for chronic diseases, translates into higher demand for lateral flow assay tests.
Download PDF Brochure:
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=167205133
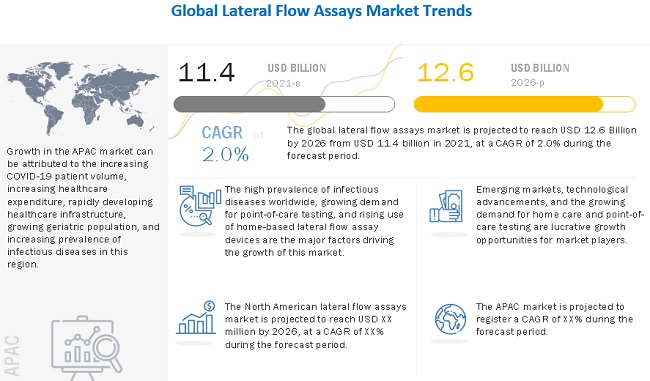
The high prevalence of infectious diseases worldwide, rapid growth in the geriatric population, growing demand for point-of-care testing, and rising use of home-based lateral flow assay devices are the major factors driving the growth of this market. However, other reluctance among doctors and patients to change existing diagnostic procedures and the low accuracy of lateral flow assays are the key factors restraining the growth of the market.
Evolving application of lateral flow assays:
Lateral flow assays have evolved rapidly in the last two decades and are routinely used in POC and diagnostic applications. Although lateral flow tests are widely being used for infectious disease diagnostics, the diagnosis of cardiac diseases, and veterinary applications, their use has increased in several new applications over the last few years. For instance, saliva diagnostics, behavioral health, agriculture (genetically modified organism detection and crop quality testing), biowarfare (anthrax detection), environmental testing (detection of contaminating enzymes in manufacturing plants), and food microbiology (detection of E. coli O157, Salmonella, Listeria, and other food spoilage organisms) have emerged as new application areas for lateral flow assays.
OraSure Technologies (US) is one of the major providers of lateral flow saliva testing. Salivary LFIAs have also penetrated the market for applications such as drugs-of-abuse testing, and several systems have been FDA-cleared for marketing; however, testing remains largely qualitative.
Salivary rapid tests have also been developed for infectious diseases. Companies such as SOMA Bioscience (UK) have developed quantitative saliva LFIAs for IgG, IgA, α-amylase, and cortisol; SOMA Bioscience’s salivary cortisol LFDs have been used in sports research. Medusa 19 Limited (UK) has developed the Rapid Saliva Protein Test (“RSPT”), which is a lateral flow saliva test to indicate the immune response to SARS-CoV-2 infection.
Request Research Sample Pages: https://www.marketsandmarkets.com/requestsampleNew.asp?id=167205133
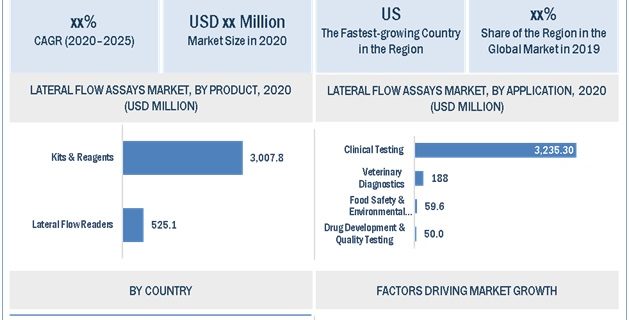
North America to dominate the Lateral Flow Assays market during the forecast period
North America is expected to account for the largest share of the Lateral Flow Assay market in 2019, followed by Europe. The dominance of the North American region can be attributed to, increasing R&D investment in the region and presence of a large number of market players.
Prominent players in the Lateral Flow Assaysmarket include Abbott Laboratories (US), F.Hoffman La-Roche Ltd. (Switzerland), Danaher Corporation(US), Becton, Dickinson, and Company (US), Siemens AG (Germany) &Thermofisher Scientific (US)