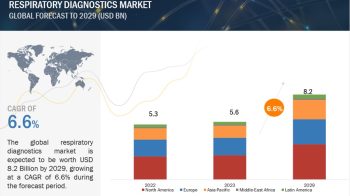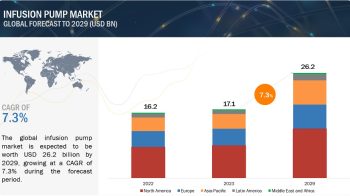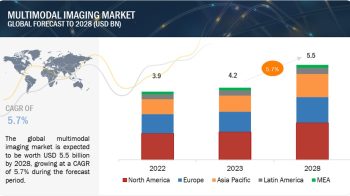
According to the new market research report ” Medical Device Reprocessing Market by Product (Reprocessed Device (Catheter, Endoscope, Laparoscopic Instruments, Biopsy, Pulse Oximeter), Service), Application (Cardiology, Gastroenterology, Arthroscopy, Orthopedic, Anesthesia) – Global Forecast “, published by MarketsandMarkets™, global medical device reprocessing market is projected to be valued at USD 823.5 Million in 2017 and is expected to grow at a CAGR of 16.3% during the forecast period to reach to USD 1,754 Million by 2022. Base year considered for the report is 2016.
[145 Pages Report] The medical device reprocessing includes cleaning, disinfection, inspection, sterilization, and repackaging of used medical devices for its reuse to diagnose and treat multiple patients.The key factors driving the growth of this market include the low prices of reprocessed devices as compared to new ones and the pressure to reduce the volume of medical waste generated.
Browse in-depth TOC on “ Medical Device Reprocessing Market”
75 – Table
24 – Figures
145 – Pages
Download PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=583
The growth of this market is majorly driven by the increased utilization of the low priced reprocessed medical devices as compared to new devices, increased efforts towards reducing regulated medical waste (RMW), and rising number of surgical procedures associated with the growing prevalence of chronic diseases. However, the risk of transmission of surgical site infections associated with the use of reprocessed devices may hinder the growth of the market to a certain extent.
The reprocessing practices are regulated by various regional regulatory authorities. The changing regulatory scenario favoring medical device reprocessing in a number of countries, such as France and Japan, will open up new areas of opportunity for market players. For example, till 2016, the outsourcing of medical device reprocessing was prohibited in France; however, the European Union (EU) Regulations (Article 17), released on April 5, 2017, mandated the use of third-party reprocessing services by all French hospitals. These regulations are expected to be implemented in France by 2020.
The report analyzes the market by product & service, type of medical device, application, and region. On the basis of product & service, the reprocessing support and services segment accounted for the largest share in 2016, owing to the increased adoption of third-party reprocessing services over in-house reprocessing services by hospitals, clinics, surgical centers, clinical pathologies, and other healthcare organizations.
Request Sample Report : https://www.marketsandmarkets.com/requestsampleNew.asp?id=583
North America to account for the largest market size during the forecast period :
Geographically, the global medical device reprocessing market is segmented into North America, Europe, Asia-Pacific, and the Rest of the World. In 2016, North America dominated the market, and this is primarily attributed to its well-established healthcare industry, growing prevalence of chronic diseases, and increasing volume of surgical procedures. The European region is expected to witness the highest CAGR during the forecast period, owing to the rising prevalence of chronic diseases, increasing geriatric population, rising healthcare expenditure, increasing number of surgical procedures, and growing awareness about the usage of reprocessed medical devices.
Key Market Players :
In 2016, Stryker Corporation (U.S.) and Johnson & Johnson (U.S.) dominated the global medical device reprocessing market. Some of the other players competing in this market are Vanguard AG (Germany), Medline ReNewal (U.S.), Medtronic plc (Ireland), SteriPro Canada, Inc. (Canada), Pioneer Medical Devices AG (Germany), Vascular Solutions, Inc. (U.S.), HYGIA Health Services, Inc. (U.S.), ReNu Medical, Inc. (U.S.), SureTek Medical (U.S.), and Centurion Medical Products Corporation (U.S.).