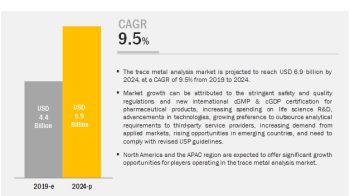
The global multiplex assays market is projected to
reach USD 3.35 Billion in 2023 from USD 2.17 Billion in 2017, at CAGR of 7.5%.
In the current market scenario, there is a growing demand for multiplex assays.
The major factors driving the growth of the market include the adoption of Companion
Diagnostics for increasing the safety & efficacy of therapies and the
advantages of multiplex assays over conventional singleplex assays.
Download PDF Brochure @
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=61593314
Opportunity: Monitoring of biosimilars in autoimmune diseases
Anti-TNF-alpha biologics are proving to be an effective treatment for
autoimmune diseases, such as rheumatoid arthritis (RA) and inflammatory bowel
disease (IBD). With the increasing healthcare costs as well as patent
expirations, the number of R&D activities focusing on the development of
biosimilars have increased over the last few years. The launch of biosimilars
in the market is increasing the need for the better understanding of potential
differences in biosimilar immunogenicity as well as their safety and efficacy
profiles as compared to their precursors. For instance, TNF-alpha blockers,
such as infliximab and adalimumab, both can trigger immunogenicity responses
and researchers have hypothesized that their biosimilars drugs (CT-P13) may
also promote immunogenicity. Therefore, it is vital to develop methods for the
TDM of biosimilar drugs for autoimmune diseases to ensure effective patient
care.
Driver: Use of TDM in traditional anticancer therapies
TDM provides valuable guidance for the dose
adjustment of antibiotics, immunosuppressants, antiepileptics, and other drugs.
However, its use in traditional anticancer therapies has been limited. The
requirement of multiple blood samples to adequately define the systemic
exposure of anticancer drugs is the major issue encountered while testing for
anticancer therapies.
Drugs in blood samples have a short elimination
half-life and are given by intermittent intravenous injections; this makes the
entire monitoring process difficult to analyze. However, newer targeted
anticancer therapies have different pharmacokinetic (PK) and dosing
characteristics as compared with traditional cytotoxic drugs. Owing to this, it
is now possible to estimate the steady-state drug exposure with a single
trough-level measurement. Recent evidence indicates that certain PK parameters,
including trough levels, are correlated with clinical outcomes for many
cytotoxic agents, including imatinib, sunitinib, rituximab, and cetuximab.
Although current evidence is not sufficient to
make TDM a compulsory practice, investigations with encouraging results will
have a practical place in the clinical care of patients with cancer. With the
increasing incidence of cancer and focus on reducing the adverse effects of
drugs, the monitoring of antineoplastic drugs is expected to gain more
importance in the treatment of cancer.
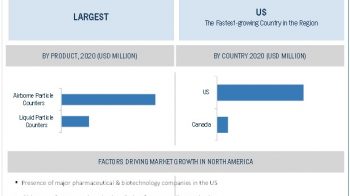
North America is expected to account for the largest market share
North
America is expected to account for the largest share of this market, followed
by Europe. Growth in the North American market is mainly driven by the
increasing per capita healthcare expenditure and the presence of
technologically advanced healthcare infrastructure in the region. Initiatives
taken by different government associations are also anticipated to boost market
growth in the coming years.
Request Sample Pages @
https://www.marketsandmarkets.com/requestsampleNew.asp?id=61593314
Key Players:
Abbott
Laboratories, Hoffmann-La Roche, Siemens Healthineers, Thermo
Fisher Scientific, Danaher Corporation, Bio-Rad Laboratories, Biomérieux,
Bühlmann Laboratories, Sekisui Medical, Randox Laboratories.