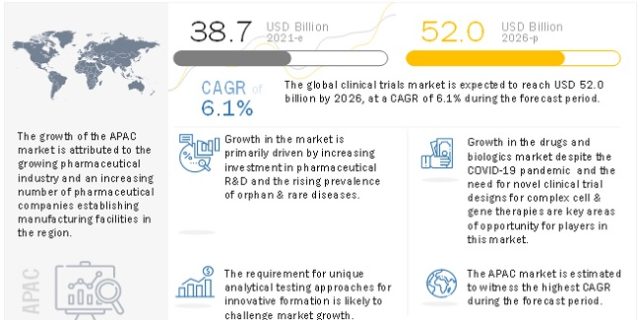
Introduction of the global Clinical Trials market:
The intangible facts surrounding the key restraints, opportunities, & risks that are anticipated to affect the industry’s development during the forecast period are investigated in the Clinical Trials Market report (2022-2026). Other assessments, like supply & demand, imports & exports, distribution networks, consumption, and production capacity, are all important in giving business owners, stakeholders, & field marketers a competitive edge over competitors in the same arena. Furthermore, the research evaluates the Clinical Trials market competitors’ flaws & strengths in several categories. For business owners to establish real business plans, they must analyse previous & future major trends which are actively contributing to the growth of the Clinical Trials industry.
A complete analysis of the global Clinical Trials industry, as well as market segmentation by product type, applications, end-use, & region, is included in the report. The report includes a historical market dynamics analysis from 2022 to 2026, which will help readers compare past trends to current market scenarios, as well as key player contributions.
Get access to a free copy of our latest sample report @
https://www.marketsandmarkets.com/requestsampleNew.asp?id=405
COVID-19 Impact on Clinical Trials market:
In the Clinical Trials market, the research also reveals exclusive choices, difficult situations, & problematic scenarios. A series of concepts will aid readers in making decisions & strategizing for their future chances. Our challenges, adversity, and market concerns let our readers realise how businesses might save them. The novel coronavirus illness (COVID-19) problem is wreaking havoc on all service & manufacturing businesses due to severe declines in demand. The majority of workforces in this arena are at risk. As an outcome, a considerable number of originalities have shut down.
The global crisis has impacted every industry. COVID-19’s market impact has been closely monitored by our analysts. A separate section of the report focuses on the setbacks which happened throughout the crisis.
Regional Insights on Clinical Trials market:
The several sections on regional segmentation provide information on the regional characteristics of the global Clinical Trials Market. This chapter discusses the regulatory environment that will have an impact on the global market. It highlights the market’s political landscape & anticipates its impact on the worldwide Clinical Trials industry. Global brands’ presence and availability, as well as the problems they face from fierce competition from local & domestic brands, the influence of domestic tariffs, and trade channels, are all considered. The Clinical Trials report examines the five areas & their distribution by country:
North America – (U.S., Canada, and Mexico)
Europe – (U.K., Germany, France, Spain, Italy, Sweden, CIS Countries, and Rest of Europe)
Asia Pacific – (China, India, Japan, South Korea, Australia, ASEAN, and Rest of APAC)
Middle East & Africa – (South Africa, GCC Countries, Nigeria, Egypt, and Rest of ME&A)
South America – (Brazil, Argentina, Colombia, and Rest of South America)
Get access to a free copy of our latest Download PDF Brochure @
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=405
Segmentation:
In 2020, the Phase III segment held the greatest proportion of the clinical trials market.
The market is divided into four phases: Phase I, Phase II, Phase III, and Phase IV. The rise of the Phase III segment has been aided by the relatively large patient population and the use of advanced research services to investigate drug efficacy. To successfully recruit patients, establish trial sites fast, and provide cost-effective and high-quality study management, these complicated clinical trials require both clinical resources and robust technologies. Clinical trial service providers are used by the majority of worldwide pharmaceutical, biopharmaceutical, and medical device businesses to conduct Phase III clinical trials.
In 2020, the laboratory services segment held the highest proportion of the clinical trials market by service type.
Protocol design, site identification, patient recruitment, laboratory services, bioanalytical testing, analytical testing, clinical trial supply & logistic services, decentralised clinical services, clinical trial data management services, medical device testing services, and other services make up the clinical trial services market. The category is expected to rise due to the growing number of clinical trial service providers offering a comprehensive range of laboratory services such as biospecimens and GMP services.
The infectious illness sector is expected to grow at the fastest rate among therapy areas over the projection period.
The clinical trials market is divided into oncology, infectious diseases, cardiology, neurology, women’s health, genetic diseases, immunology, and other therapeutic areas based on the therapy area. Because of the increased number of clinical trials for vaccines and treatments for COVID-19 and other infectious illnesses, as well as rising investments in infectious disease R&D, the infectious diseases segment is expected to grow at the fastest rate over the forecast period.
The small molecules category accounted for the greatest proportion of the clinical trials market in 2020, according to application.
The global clinical trial market is divided into small applications, vaccines, cell and gene therapy, and others based on application. Other large applications, such as hormones/insulin and blood components, are included in the others division. The solid pipeline for generic medications, which account for a significant portion of the pharma market, can be credited for the huge share of this segment. The pharma industry’s saturation with simple generic pharmaceuticals has prompted investment in complicated generic drug candidates, which is projected to propel the market forward.
Key Players:
IQVIA (US), LabCorp (US), Charles River Laboratories (US), WuXi AppTec (China), Syneos Health (US), PPD (US), and ICON Plc (US) are the prominent players operating in the clinical trials market.
Global Clinical Trials Market research report offers:
- The global Clinical Trials market is defined, along with an analysis of several influencing factors like drivers, constraints, opportunities, & challenges.
- Market risk analysis & industry trend analysis are also included in the Clinical Trials market.
- The market competition landscape is defined, characterised, & analysed using Porter’s Five Force Analysis & SWOT analysis, with a focus on global primary manufacturers.
- Identification and analysis of micro and macro aspects that influence & will influence market growth in the global Clinical Trials competitive landscape.
- A comprehensive list of key market participants in the global Clinical Trials business.
- It gives a descriptive assessment of demand-supply side analysis in the worldwide Clinical Trials market.
- A statistical analysis of certain major economic indicators
- The market is described using figures, charts, graphs, & drawings.
Will You Have Any Questions About This Report? Please Contact Us On:
https://www.marketsandmarkets.com/Market-Reports/clinical-trials-market-405.html
https://www.marketsandmarkets.com/PressReleases/clinical-trials.asp