This report aims to provide detailed insights into the Point-of-Care Molecular Diagnostics Market. It provides valuable information on the type, procedure, application, and region in the market. Furthermore, the information for these segments, by region, is also presented in this report. Leading players in the market are profiled to study their product offerings and understand the strategies undertaken by them to be competitive in this market.
Revenue Growth Analysis:
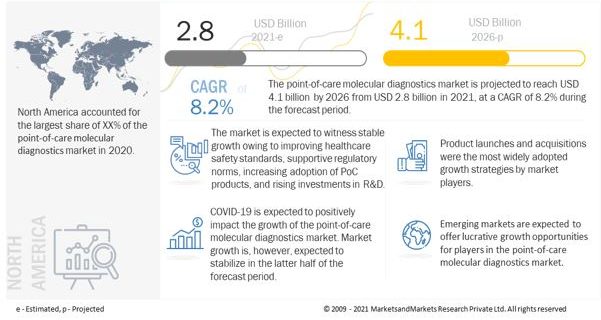
[233 Pages Report] The global point-of-care molecular diagnostics market size is projected to reach USD 4.1 billion by 2026 from USD 2.8 billion in 2021, at a CAGR of 8.2%
Download PDF Brochure:
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=143524127
Key Factors Driving Market Growth:
Market growth is driven by the increasing prevalence of infectious diseases and cancer, rising focus on decentralized diagnostics, increasing R&D funding, increasing awareness on the early detection of infectious diseases, and the increasing use of POC diagnostic tests.
Increasing Prevalence of Infectious Diseases and Cancer
The increasing prevalence of infectious diseases and cancer in developed and developing regions will positively influence the growth of the point-of-care molecular diagnostics market. The diagnosis and management of such diseases are responsible for the increasing number of prescriptions for molecular diagnostic tests. These factors, alongside the growing trend for preventive medicine, is expected to drive the demand for point-of-care molecular diagnostics during the forecast period.
Growth Opportunities in Emerging Countries
Emerging economies such as India, South Korea, Brazil, and Mexico offer significant growth opportunities to players operating in the point-of-care molecular diagnostics market. This can be attributed to the low regulatory barriers, improvements in healthcare infrastructure, growing patient population, rising prevalence of infectious diseases, and rising healthcare expenditure. Moreover, the regulatory policies in some of these countries are more adaptive and business-friendly than those in developed countries.
RT-PCR Segment Accounted for the Largest Share in the Market, by Technology
The point-of-care molecular diagnostics market is segmented into RT-PCR, INAAT and other technologies based on technology. In 2020, the RT-PCR segment accounted for the largest share. This can be attributed to the growing use of RT-PCR in proteomics, genomics, and COVID-19 testing as well as the access to portable, easy-to-use devices are the major factors driving the growth of this market segment.
North America is expected to dominate the POC Molecular Diagnostics Market
The global point-of-care molecular diagnostics market is segmented into North America, Europe, Asia Pacific and Rest of the World. In 2020, North America accounted for the largest share of the global market. The North American point of care molecular diagnostics market’s growth can be attributed to the highly developed healthcare system in the US and Canada and the easy accessibility to technologically advanced instruments.
Request Sample Pages:
https://www.marketsandmarkets.com/requestsampleNew.asp?id=143524127
The major players in point-of-care molecular diagnostics market are Abbott Laboratories (US), F. Hoffmann-La Roche Ltd. (Switzerland), bioMérieux SA (France), Danaher Corporation (US), Quidel Corporation (US), QIAGEN N.V. (Netherlands), Co-Diagnostics, Inc. (US), Biocartis NV (Belgium), Meridian Bioscience, Inc. (US), Thermo Fisher Scientific, Inc. (US), Lucira Health, Inc. (US), Cue Health (US), OpGen, Inc. (US), Binx Health, Inc. (US), Molbio Diagnostics Pct. Ltd. (India), Genomadix (Canada), Visby Medical, Inc. (US), QuikPath PTE Ltd. (Singapore), MD-Bio (US), QuantuMDx Group Ltd. (UK), Aidian Oy (Finland), and GeneSTAT Molecular Diagnostics, LLC (US).