Risk Based Monitoring (RBM) is a clinical
trial-monitoring technique that fulfills regulatory requirements
but moves away from 100% source data verification (SDV) of patient data. It employs various tools, platforms and dashboards
to identify signals, which indicate potential issues with (for example) trial
conduct, safety, data integrity, compliance and enrolment.
What The Market Looks Like?
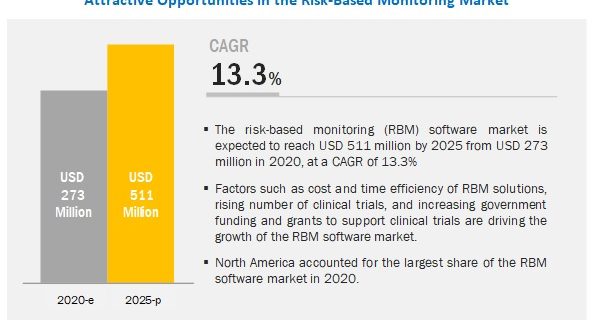
The risk-based
monitoring (RBM) software market is expected to reach USD 511 million by
2025 from USD 273 million in 2020, at a CAGR of 13.3%.
Based on the component, the RBM software market is segmented into Software and
Services. In 2020, the software segment is expected to command the largest
share of the RBM software market. The large share of this segment can be
attributed to can be attributed to the increasing R&D expenditure in the
life science and clinical research industries, an increasing number of clinical
trials, and rising customer base.
The pharmaceutical and biopharmaceutical
companies segment is expected to command the largest share of the RBM software
market. The increasing R&D expenditure of pharmaceutical and
biopharmaceutical companies is the major factor driving the growth of this end-user
segment.
Download PDF Brochure:
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=102112429
Key Factors Driving Growth in RBM Software Market:
1. Cost and time efficiency of RBM solutions
2. Rising number of clinical trials
3. Increasing government funding and grants to support clinical trials
4. Increased outsourcing of clinical trial processes to CROs
Geographical Growth Analysis of Risk-based Monitoring Software Market:
The Asia Pacific market is expected to grow at the highest CAGR in the forecast
period. This growth can be attributed to increasing government funding to
support clinical trials, the presence of less stringent regulatory guidelines
as compared to developed nations, a large patient base, low operating costs for
conducting clinical trials, shortage of trial volunteers in Europe and North America,
and the growing number of pharmaceutical companies and CROs in the region.
Read more:
https://www.marketsandmarkets.com/Market-Reports/risk-based-monitoring-software-market-102112429.html