Factors driving the growth of this segment include increasing adoption of urology devices in hospitals and clinics, rising incidence of chronic disorders, and increasing demand for minimally invasive surgeries in urology.
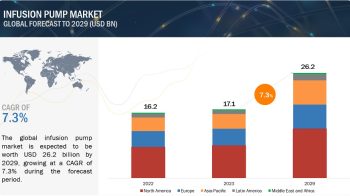
The Urology Devices Market is expected to reach USD 44.37 Billion by 2022 from USD 31.44 Billion in 2017, at a CAGR of 7.1%.
Download PDF Brochure:-https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=173062212
By disease, the market is segmented into kidney disease, urological cancer and BPH, pelvic organ prolapse. The kidney diseases segment is expected to account for the largest share of the global urology devices in 2017. A large number of products are used in the diagnosis and treatment of this segment which is a major factor responsible for the dominant share of this segment.
By product, the urology devices market is classified into instruments and consumables & accessories. The instruments segment is expected to lead the global urology devices market in 2017.
On the basis of end user, the urology devices market is categorized into hospitals and clinics, dialysis centers, and other end users. The hospitals and clinics segment is estimated to account for the largest share of the global urology devices market during the forecast period. The heavy burden of urological cancers such as prostate cancer and bladder cancer, along with the accessibility and availability of diagnosis and treatment instruments in hospitals and clinics in a short period of time are the key factors driving the growth of this end-user segment.
Based on region, the urology devices market is segmented into North America, Europe, Asia, and the Rest of the World (RoW). North America is expected to dominate the urology devices market in 2017.
Read More: – https://www.marketsandmarkets.com/PressReleases/urology-devices.asp
Key players in the urology devices market include Fresenius Medical Care (Germany), Baxter (US), Boston Scientific (US), Olympus (Japan), Richard Wolf (US), KARL STORZ (Germany), Cook Medical (US), Medtronic (US), C. R. Bard (US), Dornier MedTech (Germany), Prometheus Group (US), Medi-Globe (US), Intuitive Surgical (US), Merit Medical Systems (US), Siemens Healthcare (Germany), Stryker (US), ROCAMED (France), Medica (Italy), NOVAmedtek (Turkey), SRS Medical Systems (US), ProSurg (US), Albyn Medical (UK), EMD Medical Technologies (Canada), and Biolitec (Germany).