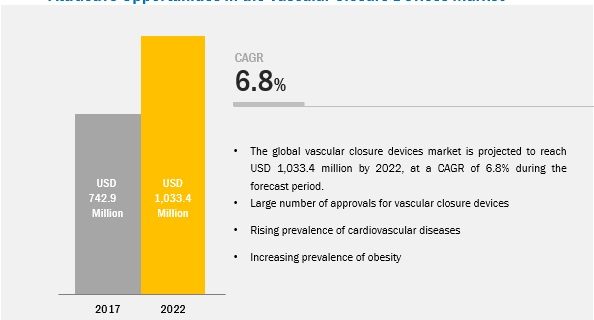
The major drivers for this market include the large number of approvals for vascular closure devices, the high prevalence of cardiovascular diseases, high prevalance of obesity, and increasing focus of market players on large-bore vascular closure devices.
The global vascular closure devices market is expected to reach USD 984.6 Million by 2022 from USD 704.8 Million in 2017, at a CAGR of 6.9%. Vascular Closure Devices Market by Type (Passive Approximators (Collagen Plugs Sealent/Gel Based), Active Approximators (Clip, Suture Devices)), Access (Femoral,Radial) & Procedure (Interventional Cardiology, Radiology) – Global Forecasts to 2022.
In this report, the market has been segmented based on type, access, procedures, and geographies. The type segment includes passive approximators, active approximators, and external hemostasis devices. Passive approximators are expected to account for the largest share of the market in 2017. Passive approximators are the most widely used vascular closure devices in the market owing to the wide range of advantages associated with them, including increased safety and fewer complications than manual procedures.
The access segment includes femoral access and radial access. The femoral access segment is estimated to account for the largest share of the market in 2017 owing to the widespread preference for this access route among cardiologists and physicians during interventional procedures as well as the fewer complications involved during arterial access.
The procedures segment of the vascular closure devices market includes interventional cardiology and interventional radiology/vascular surgery. The interventional cardiology segment is expected to hold the largest share of the market in 2017. The large share of this segment can primarily be attributed to the high prevalence of cardiovascular diseases and increasing obese population across the globe.
Download PDF Brochure:
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=180101468
Vascular closure devices are relatively high-priced devices compared to manual compression devices. The average price of a vascular closure device is about USD 150–USD 250 while that of a manual compression device is about USD 15–USD 25. The cost of VCDs essentially adds up to the patient’s hospitalization bill and even increases the per patient cost for hospitals and simultaneously reduces profits.
Moreover, radial artery access makes it more convenient for doctors to achieve hemostasis using simple manual compression methods. Interventional procedures using radial artery access do not generally require vascular closure devices, which is another factor that leads to cost savings.
Additionally, malfunctioning of devices, poor quality, and underperformance of devices are the primary causes of product recalls. Product failures and recalls cost companies millions of dollars in product replacements and lost revenues. They may also create a negative impression about the product, which in turn may limit the adoption of these products.
Region Covered in Vascular Closure Devices Market
The geographic segments in this report include North America, Europe, Asia-Pacific, and RoW. Of these, the North American segment is expected to account for the largest share of the market in 2017. The large share of this segment can primarily be attributed to the high prevalence of cardiovascular diseases in the U.S., growing trend of one-day surgery for vascular procedures, and increasing research and clinical trials for vascular closure devices.
Request for Sample Pages:
https://www.marketsandmarkets.com/requestsampleNew.asp?id=180101468
Key Players in Vascular Closure Devices Market
The major players in Vascular Closure Devices Market include Terumo Corporation (Japan), Abbott (U.S.), Cardinal Health Inc. (U.S.), Cardiva Medical Inc. (U.S.), Morris Innovative, Inc. (U.S.), Medtronic plc (Ireland), Essential Medical, Inc. (U.S.), Merit Medical Systems, Inc. (U.S.), TZ Medical, Inc. (U.S.), and Vasorum Ltd. (Ireland).
