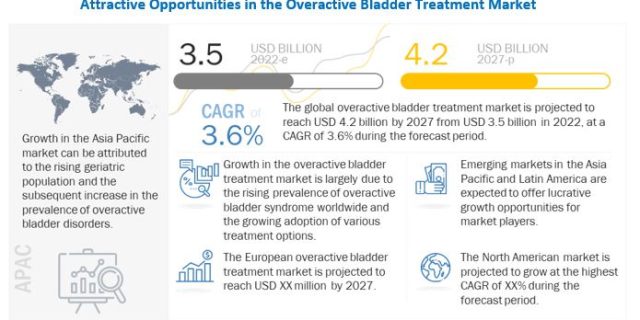
According to the forecast, the global Overactive Bladder Treatment Market is expected to expand from USD 3.5 billion in 2022 to USD 4.2 billion by 2027, showcasing a Compound Annual Growth Rate (CAGR) of 3.6% during the projected period. The substantial growth of this market can be attributed to several key factors. Firstly, there is a notable increase in the prevalence of overactive bladder syndrome worldwide, which is driving the demand for effective treatments. Additionally, the introduction of novel medications that offer reduced side-effects, along with the adoption of technologically advanced treatment options specifically designed for OAB, is expected to further facilitate the market’s growth in the forthcoming years.
Download PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=219938791
DRIVER: Growing R&D investments and the launch of novel therapies in the coming years
Currently, there is a considerable investment from biopharmaceutical and pharmaceutical firms in developing groundbreaking therapies aimed at treating overactive bladder. These innovative treatments are projected to be introduced in the foreseeable future. An example of such progress can be seen in Taiho Pharmaceutical’s efforts, as they are actively working on a drug named TAC-302, which is currently undergoing Phase II development. TAC-302 is an orally-administered drug that has the potential to effectively address overactive bladder by stimulating the growth of nerve fibers in peripheral neurons that have been cultured. With the introduction of these novel treatment options, there is an expected rise in the acceptance and utilization of these treatments, consequently fostering the expansion of the market.
OPPORTUNITY: Novel treatments, robust pipelines, and patent cliff of certain drugs
Currently, there is an extensive range of over 30 potential treatments for overactive bladder (OAB) being evaluated in clinical trials. Among these candidates, three are in the Phase II stage, seven are in Phase III, and sixteen are in Phase IV. This robust pipeline of ongoing research and the subsequent introduction of new medications and therapies are anticipated to be key drivers of growth in the market for treating overactive bladder. In an effort to enhance understanding and treatment efficacy, Samsung Medical Center is conducting a study on OAB patients, examining potential biomarkers such as Nerve Growth Factor (NGF), prostaglandin E2 (PGE2), and adenosine triphosphate (ATP), as well as the impact of various drugs on these biomarkers. The aim is to identify indicators that can help predict the responsiveness of patients to specific treatments, enabling the provision of personalized and tailored therapeutic approaches. These advancements hold significant potential for fostering substantial growth opportunities for stakeholders operating within the overactive bladder treatment market.
Overactive bladder treatment market is fragmented and competitive in nature, several market players are adopting strategies to sustain market competition. The company offerings range from, various drugs, devices and botox that have larger geographic reach along with high product flexibility. Prominent players in the global overactive bladder treatment market are Astellas Pharma (Japan), Teva Pharmaceutical Industries (Israel), Pfizer (US), Medtronic (Ireland), AbbVie (US), Viatris (US), Hisamitsu Pharmaceutical (Japan), Johnson & Johnson Services (US), Endo Pharmaceuticals (Ireland), and among others.
Astellas Pharma is a Japanese multinational pharmaceutical company formed from the merger of Yamanouchi Pharmaceutical Co. and Fujisawa Pharmaceutical Co. Astellas Pharma is engaged in the research, development, manufacturing, import, and export of pharmaceutical products. It is one of the leading players operating in the overactive bladder treatment market globally. The company provides two major drugs—Betanis/Myrbetriq/BETMIGA and VESIcare. Myrbetriq is one of the most prescribed medications for overactive bladder disorders. The company has its research & development base in Japan and R&D centers in Europe and other countries across the Asia Pacific. The company operates in more than 70 countries across the globe.
Request Sample Pages: https://www.marketsandmarkets.com/requestsampleNew.asp?id=219938791
Pfizer is a leading global pharmaceutical company. It develops medicines for a wide range of therapeutic areas, such as metabolic & cardiovascular diseases, inflammation & immunology, neuroscience & pain, oncology, rare diseases, and vaccines. The company operates through three distinct business segments: Biopharma, Pfizer CentreOne, and Consumer Healthcare. In July 2019, the Consumer Healthcare business, an OTC medicines business, was combined with GSK’s Consumer Healthcare business to form a new Consumer Healthcare JV.
Related Links
https://www.marketsandmarkets.com/Market-Reports/overactive-bladder-treatment-market-219938791.html
https://www.marketsandmarkets.com/ResearchInsight/overactive-bladder-treatment-market.asp
https://www.marketsandmarkets.com/PressReleases/overactive-bladder-treatment.asp

