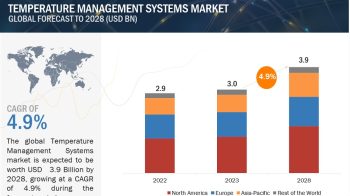
The global PCR technologies Market is expected to reach USD 18.3 billion by 2027 from an estimated USD 12.5 billion in 2022, at a CAGR of 8.0% from 2022 to 2027.
Polymerase chain reaction (PCR) is a well-established technique used for the in vitro amplification of a given nucleotide (such as DNA). This technique is used to prepare multiple copies of a gene and to synthesize a large number of DNA molecules from a given DNA sample. This is done by separating the two strands of the DNA, marking its location with a primer, and using a DNA polymerase enzyme to synthesize and assemble a copy of the gene alongside a template.
Download a PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=96291811
PCR Technologies Market by Technique (Conventional, qPCR, dPCR), Product (Instrument, Reagents, Software), Application (Genotyping, Sequencing, Gene Expression, Diagnostics), End User (Academia, Pharma-Biotech, Applied) – Global Forecast to 2027
Growth in this market is largely driven by factors such as the rising incidence of target infectious diseases and genetic disorders, continuous advancements in PCR technologies and increasing investments.
The demand for early disease diagnosis and treatment has driven the R&D in developing genetic technologies and product development. This rise in R&D activity is supported by an increase in investments and the availability of funds and grants. For instance, in March 2018, vc (WGGC) and the Universities of Cologne, Bonn, and Düsseldorf (Germany), received ~USD 5.98 million (EUR 5.3 million) for a joint competence center from the German Research Foundation (DFG).
Government and federal agencies across the globe are recognizing the role of qPCR and dPCR products (such as instruments, reagents, and software) in genomics-based research. In the last decade, the qPCR and dPCR market has witnessed several funding initiatives by government and private agencies to support genomic research. In October 2020, Visby Medical (US), an infectious disease diagnostic company, received Phase 2 funding from the National Institutes of Health (NIH) as part of its Rapid Acceleration of Diagnostics (RADx) initiative. In addition, Centre d’innovation Génome Québec et Université McGill (Canada) received ~USD 10 million in funding from federal and provincial investments on February 2019. The funding will be utilized for projects in the field of bioinformatics, epigenomics, and precision medicine, among other fields (Source: McGill University, Canada). These initiatives are aimed at conducting medical genomics-based studies in personalized medicine, including PCR-based disease diagnosis and treatment.
Thus, with the growing availability of financial support for sustaining R&D in disease-targeted genomics research activities, the demand for PCR products is expected to increase in the coming years.
On the other hand, issues related to high instrument costs of dPCR and the limitations of PCR technologies are expected to restrict the market growth to a certain extent in the coming years.
dPCR instruments integrates scientific technologies such as PCR, microfluidics, and nanofabrication, to conduct a typical PCR analysis. These instruments are small, however, their development requires big investments and extensive scientific validation on a nanoscale level. As of 2022, the average price of a dPCR instrument in the US is reported to be USD 55,000–120,000. Such high prices have resulted in the subdued adoption of dPCR instruments among price-sensitive end users. Moreover, the dPCR instruments are expensive compared to qPCR instruments (~USD 90,000).
Due to the preference for cheaper genomic techniques (such as gel electrophoresis and traditional PCR), the demand for premium-priced dPCR instruments and reagents is restricted, especially among researchers and small CROs and pharmaceutical & biotechnology companies.
On the basis of product, the global PCR technologies market is broadly classified into instruments, reagents and consumables, and software and services. In 2021, the reagents and consumables segment accounted for the largest share of the PCR technologies market. This is mainly due to the expanding applications of dPCR, increasing number of dPCR-based analytical procedures among institutional researchers (such as CROs and pharma-biotech companies), and the rising availability of novel dPCR reagents worldwide. Additionally, the expansion of the application base of the dPCR technology in genetic/molecular research, clinical diagnosis, and forensics is expected to result in the commercialization of novel dPCR reagents.
The growth of reagents and consumables segment is majorly due to sale of PCR reagents through various approaches by prominent players (such as bundled sales of PCR reagents for 3–5 years along with the purchase of PCR instruments; long-term sales contracts for PCR reagents upon device upgradation; and bundled sales of related consumables on the long-term purchase of PCR reagents) driving the demand and adoption of PCR reagents and consumables in major markets such as Europe, the US, Japan, China, and India.
The growth of the PCR reagents and consumables market will be driven by the expanding applications of dPCR, increasing number of dPCR-based analytical procedures among institutional researchers (such as CROs and pharma-biotech companies), and the rising availability of novel dPCR reagents worldwide.
The continuous decline in the costs of qPCR reagent (owing to the greater market competition and pricing pressure) are expected to restrain the growth of this market during the forecast period.
The major end users of PCR technologies include hospitals and diagnostic centers, healthcare industry, academia and government organizations, pharma-biotech companies, applied industries, and other end users. The hospitals and diagnostic centers segment accounted for the largest share of the PCR technologies market in 2021. The increasing market availability of qPCR reagents for clinical diagnosis, ongoing expansion of healthcare infrastructure in emerging APAC and Eastern European countries, and the continuous reduction in the cost of qPCR reagents are some of the factors driving the segment.
The hospitals and diagnostic centers segment comprises in-house diagnostic and clinical research centers within hospitals, intendant diagnostic and pathology centers, and reference laboratories (which work for small hospitals, independent physicians, and community clinics). These end users use techniques such as qPCR and dPCR for the diagnosis of target diseases and disorders (such as cancer, HIV-AIDS, genetic disorders, infectious diseases, and other chronic diseases).
In hospitals and diagnostic centers, PCR technologies are used for viral load testing, genetic testing, profiling of embryo DNA before in vitro fertilization, and organ/tissue transplant procedures. The increasing market availability of qPCR reagents, expansion of healthcare infrastructure across emerging countries, high prevalence and incidence of target diseases, and the growing awareness among end users related to the benefits offered by qPCR in rapid and effective disease diagnosis are some of the major factors driving market growth in this end-user segment.
However, the high costs associated with PCR products (including instruments, reagents, and software) and the limited availability of specific PCR reagents and assays for the clinical diagnosis of infectious diseases and blood screening are expected to restrain the growth of this market during the forecast period.
On the basis of technique, the PCR technologies market is segmented into conventional PCR, real-time PCR (qPCR), digital PCR (dPCR), reverse transcription PCR (RT-PCR), hot-start PCR, multiplex PCR, and other PCR techniques. The qPCR segment accounted for the largest share of the market in 2021. Factors such as the expanding applications of qPCR (owing to its technological benefits over traditional PCR, such as real-time analysis and reduced analysis time), growing private-public funding for life sciences research, and the rising number of probe-based multiplex genetic analysis procedures (that require the analysis of low-volume gene samples) are expected to drive the growth of the qPCR market.
The qPCR technique has several benefits over traditional PCR, such as an increased dynamic range of target detection, ability to collect data during the exponential phase of the reaction, no requirement for post-procedure processing, real-time monitoring, time efficiency, and kinetics measurement in the early phases of the reaction.
The qPCR segment dominates this market owing to factors such as the ongoing automation of laboratory techniques (including the integration of robotics with qPCR); growing acceptance of qPCR among researchers and healthcare professionals during and post Covid-19; and increased funding, grants, and public-private investments for qPCR-based research. Over the last few years, a significant surge in the adoption of technologically advanced qPCR instruments has been witnessed owing to the various procedural benefits offered by them. For instance, robotic qPCR devices offer several benefits as compared to traditional qPCR instruments, including reduced procedural costs, lower reaction time, minimized manual errors, improved productivity, and no risk of external contamination.
However, the growing acceptance of dPCR in genomics research and the availability of alternative technologies for clinical diagnosis will restrain the growth of the qPCR market to a certain extent during the forecast period. Furthermore, a major challenge to existing market players is the entry of a large number of small-scale and local reagent manufacturers in emerging countries.
The global PCR technologies market is segmented into North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa. In 2021, North America accounted for the largest share of 36.6% of the global PCR technologies market, followed by Europe. Growth in the North American PCR market is expected to be driven by factors such as increasing funding and grants to develop innovative PCR technologies and rising number of genome-based drug development activities.
On the other hand, the Asia Pacific is expected to grow at the fastest CAGR during the forecast period. The high growth of this segment is mainly attributed to the ongoing expansion & modernization of healthcare infrastructure in emerging Asian countries, increasing number of research projects, rising outsourcing of clinical research to Asia-based CROs by leading drug manufacturing companies, and continuous government support for genomics-based research activities in emerging Asia Pacific countries.
Factors such as the sustained economic growth in several Asia Pacific countries have resulted in significant growth in healthcare spending in this region. Moreover, increasing penetration of diagnostic technologies (including dPCR and qPCR) for research and clinical applications, growing awareness among physicians and healthcare professionals about the benefits of gene-based disease diagnosis and treatment, and the increasing public-private support to develop novel PCR technologies in Asia Pacific countries are expected to support market growth in the region during the forecast period.
However, the high cost of PCR instruments and lack of skilled technicians are expected to limit the adoption of these instruments in price-sensitive Asia Pacific markets in the coming years.
Prominent players in the PCR technologies market include F. Hoffman-La Roche Ltd. (Switzerland), Thermo Fisher Scientific, Inc. (US), Bio-Rad Laboratories, Inc. (US), QIAGEN N.V. (Netherlands), Takara Bio, Inc. (Japan), Agilent Technologies, Inc. (US), bioMérieux S.A. (France), Fluidigm Corporation (US), Danaher Corporation (US), Abbott Laboratories (US), Merck KGaA (Germany), Becton, Dickinson and Company (US), Promega Corporation (US), Eppendorf AG (Germany), and Analytik Jena AG (Germany).
Other players in the market include Meridian Bioscience Inc. (US), Stilla Technologies (France), JN Medsys (Singapore), Avance Biosciences Inc. (US), LGC Biosearch Technologies (UK), Azura Genomics Inc. (US), Bioneer Corp. (South Korea), Blue-Ray Biotech (Taiwan), Luminex Corporation (US), Bio Molecular Systems (Australia), Azure Biosystems (US), Chai Inc. (US), Enzo Life Sciences (US), Biomeme Inc. (US), and ELITech Group SAS (France).
Recent Developments
- In May 2022, Bio-Rad Laboratories, Inc. launched its CFX Duet Real-time PCR System in select countries accepting the CE Mark. The system is to support researchers in developing singleplex and duplex quantitative PCR (qPCR) assays
- In January 2022, QIAGEN N.V. and Atila BioSystems collaborated that extend the QIAcuity ecosystem to provide non-invasive prenatal testing (NIPT) solutions to QIAGEN’s dPCR franchise.
- In January 2021, Danaher Corporation announced that Health Canada issued Cepheid a medical device license for Xpert Xpress SARS-CoV-2/Flu/RSV