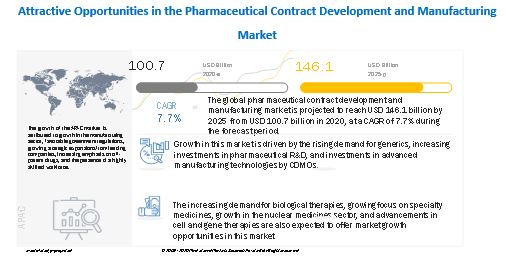
Rising demand for generics, increased investments in pharmaceutical R&D, and CDMO investments in innovative manufacturing technologies are all driving market expansion. In the future years, market expansion is likely to be fueled by rising demand for biological therapies, a growing focus on specialty medications, growth in the nuclear medicine industry, and advancements in cell and gene therapies.
[253 PAGES] The global pharmaceutical contract development and manufacturing market is expected to grow at a CAGR of 7.3 percent from USD 120.6 billion in 2021 to USD 171.3 billion in 2026.
Download PDF Brochure:
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=201524381
Impact of COVID-19 on pharmaceutical contract development and production
Companies have increased their R&D and manufacturing efforts to develop and deliver vaccines and treatments against the SARS-CoV-2 virus in response to the Covid-19 pandemic. Vaccine-related research in pharmaceutical and biotechnological firms, research centres, and academic research institutes is regarded critical, and operations and output have been largely unaffected. Many pharmaceutical and biotechnology businesses partnered with CROs and CDMOs through long-term agreements, partnerships, and collaborations all over the world to speed up the R&D and manufacturing process.
DRIVER: Patent expiration and rising generic medication demand
Generics are low-cost medications having therapeutic efficacy and safety profiles similar to those of their branded counterparts. One of the primary factors fueling the expansion of the generics industry is the increasing push to reduce rising healthcare costs. Because of these cost savings, governments around the world are supporting the use of generic medications.
OPPORTUNITY: The demand for cell and gene therapies is increasing.
Cell and gene therapies are very particular due to their tailored character, and they have the ability to meet unmet medical requirements related with treating a variety of illnesses. Many pharmaceutical companies and investors have invested enormous resources in developing and commercialising these medicines because of their strong therapeutic potential. The FDA has approved nine cell and gene therapies as of February 2020. Around 362 cell and gene treatments were in clinical trials by 2020. The expanding number of cell therapy candidates, combined with their quick progression through various stages of clinical development and their complex manufacturing process, is driving increased demand for facilities that provide these therapies’ manufacturing services.
Request For Sample:
https://www.marketsandmarkets.com/requestsampleNew.asp?id=201524381
Key Market Players
Key players in the pharmaceutical contract development and manufacturing market include Thermo Fisher Scientific Inc. (US), Catalent, Inc. (US), Lonza Group Ltd. (Switzerland), Recipharm AB (Sweden), AbbVie Inc. (US), Aenova Group (Germany), Almac Group (UK), and Siegfried Holding AG (Switzerland)
More Information:

