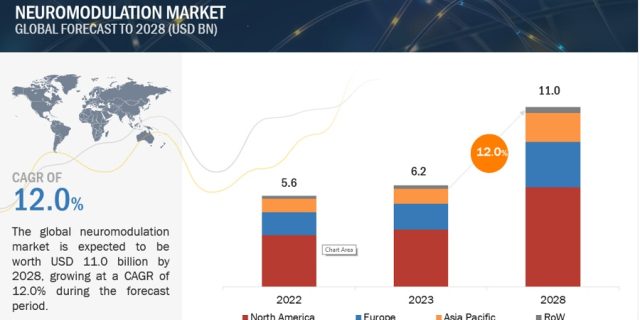
The global neuromodulation market is projected to reach USD 11.0 billion by 2028 from USD 6.2 billion in 2023, at a CAGR of 12.0% during the forecast period. The neuromodulation market is driven by increasing focus on the development of advanced neuromodulation and neurostimulation technologies, rising prevalence of neurological disorders and nerve injuries, government support for research on neurological disorders, availability of reimbursement of neuromodulation devices, collaborations among device manufacturers, healthcare providers, and research institutions to develop neuromodulation devices.
The rising incidences of neurological diseases across the globe have increased significantly. Neuromodulation is used to treat and improve the quality of life of individuals suffering from chronic illness.
Download a PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=921
Neuromodulation Market by Type (Internal (Spinal Cord Stimulation, Deep Brain Stimulation), External (Transcranial Magnetic Stimulation), Application (Tremor, Epilepsy, Parkinson’s Disease, Depression, Gastroparesis), and Region – Global Forecast to 2028
On the basis of type, neuromodulation market is segmented into internal neuromodulation and external neuromodulation. In 2022, internal neuromodulation accounted for the largest share of the global neuromodulation market. This growth can be mainly attributed to reduction of post surgical complications, reduced hospitalizations, and long term cost savings associated with internal neuromodulation devices.
The internal neuromodulation market is further categorized into sacral nerve stimulation, spinal cord stimulation, vagus nerve stimulation, deep brain stimulation, and gastric electrical stimulation. The spinal cord stimulation segment accounted for the largest share of the neuromodulation market in 2022, owing to the increasing number of people suffering from failed back surgery syndrome, ischemia, and chronic pain as well as the favorable reimbursement scenario for spinal cord stimulation.
The external neuromodulation market is segmented into transcutaneous electrical nerve stimulation (TENS), transcranial magnetic stimulation (TMS), and respiratory electrical stimulation (RES). In 2022, transcutaneous electrical nerve stimulation will dominate the external neuromodulation market. This is mainly due to the higher prevalence of depression, its chronic and recurrent nature, and the life-shortening effects of this disease, which have created a demand for TENS therapy.
Based on application, the neuromodulation market has been segmented into vagus nerve stimulation, spinal cord stimulation, deep brain stimulation, sacral nerve stimulation, gastric electrical stimulation, transcutaneous electrical nerve stimulation, and transcranial magnetic stimulation. The neuromodulation market for Spinal cord stimulation by application is further segmented into chronic pain, failed back syndrome and ischemia. In 2022, chronic pain applications dominated neuromodulation market. This growth can mainly attributed to the growing aging population and subsequent increase in prevalence of neurological disorders.
The neuromodulation market is categorized into four major regions: North America, Europe, the Asia Pacific, and Rest of the World. The Asia Pacific market is estimated to grow at the highest CAGR during the forecast period, primarily due to the increasing public and private investments, increasing incidence of neurological disorders and ongoing research.
Major Players:
Medtronic (Ireland), Boston Scientific Corporation (US), Abbott Laboratories (US), LivaNova (UK), NeuroPace (US), Neuronetics (US), Helius Medical Technologies (US), Renishaw (UK), GTX Medical (Netherlands), Bioinduction (UK), Axonics (US), Nevro Corporation (US), Nalu Medical (US), Aleva Neurotherapeutics (Switzerland), GiMer Medical (Taiwan), Magtism (UK), NeuroSigma (US), BioWaveGo USA (US), Synapse Biomedical (US), BIOTRONIK (Germany), Theranica Bio-Electronics (Israel), MicroTransponder (US), Soterix Medical (US), Saluda Medical (US), Electrocore, Inc. (US) and tVNS Technologies (Germany)
Recent Developments of Neuromodulation Industry:
- In 2023, Medtronic (Ireland) received CE (Conformité Européenne) Mark approval for its Inceptiv closed-loop rechargeable spinal cord stimulator (SCS).
- In 2023, Boston Scientific Corporation (US) received the US FDA approval for the Vercise Neural Navigator 5 Software/ It is a part of the Vercise Genus Deep Brain Stimulation (DBS) Systems.
- In 2023, Boston Scientific Corporation (US) entered into an agreement to acquire Relievant Medsystems, Inc. (US) to expand its neuromodulation portfolio to provide more treatment options for people living with chronic low back pain.
- In 2022, Medtronic (Ireland) received FDA approval for InterStim X System, which is a next-generation personalized sacral nerve stimulation therapy used for bladder and bowel control.
![Pen Needles Market Size, Share, Trends and Revenue Forecast [2028]](https://mnmblog.org/wp-content/uploads/2024/07/pen-needles-market-350x196.jpg)